Ultragenyx Pharmaceutical
FDA Approves Crysvita for Rare Rickets
The FDA approved Crysvita, the first drug indicated for adults and children aged 1 year and older with X-linked ...
APRIL 18, 2018
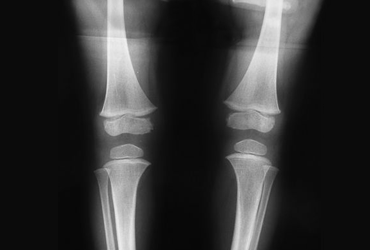
FDA Approves Mepsevii for Rare Genetic Enzyme Disorder
MPS VII is a rare lysosomal storage disorder caused by deficiency of the beta-glucuronidase enzyme, an enzyme ...
NOVEMBER 15, 2017
Load more
